Suscribe to the linkedin page of Amypore
The latest news about our therapeutic molecule AmyP53 !
website amypore.com

Un roman scientifique de haut vol
L'ADN perdu: le secret de la Vie dans l'Univers
Disponible sur Amazon (bénéfices de l'auteur versés à une association d'aide aux enfants hospitalisés)
déjà 120€ versés
à Sourire à la Vie !
Hypothèse stupéfiante sur l'origine de la vie !
Je recommande +++
Informations
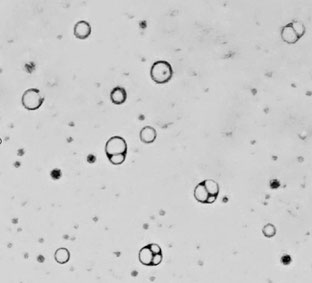
Les étudiants de la Licence Sciences de la Vie à l'Université d'Aix-Marseille (L3 Parcours PGF) rivalisent de talent pour fabriquer des vésicules prébiotiques en séance de Travaux Pratiques.
Voici une magnifique image de ces vésicules préparées avec seulement 3 composés issus de météorites :
de l'eau, de l'acide nonanoïque et du nonanol.
Travail d'Eva BARASTON, Sofiane BENHAMED et Sarah BRAHIMI (5/12/2023)
UE Origine de la Vie & Evolution Moléculaire
Observation au microscope optique
Expérience réalisée selon la méthode de David Deamer :
AmyP53 therapeutic innovation
for Alzheimer and Parkinson
Video explanation of AmyP53 technology.
© Christelle GOBET, a talented student in Neurobiology
in english:

and in french:

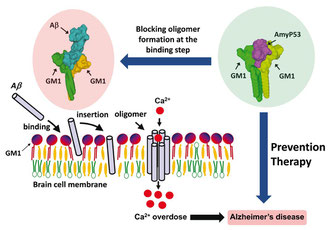
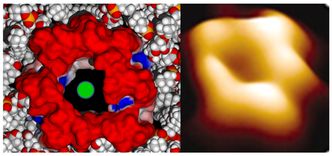
Molecular modeling of amyloid pores (J. Fantini)
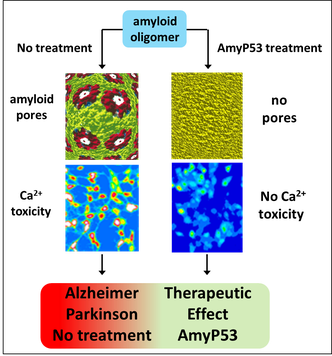
Amyloid plaques are no longer regarded as the pathogenic forms of Alzheimer's beta amyloid peptide (Abeta). Instead, small oligomers of amyloid proteins (Abeta and alpha-synuclein) that form Ca2+ permeable channels (amyloid pores) are now considered as the most neurotoxic species of amyloid proteins in neurodegenerative disorders. By disrupting Ca2+ homeostasis in brain cells, amyloid oligomers induce dramatic synaptic dysfunctions, impaired plasticity and neuronal degeneration. Hence, the oligomer model has all but supplanted the classical amyloid cascade.
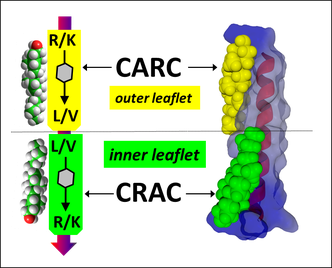
Our group demonstrated that amyloid pores are formed by a common mecanism that can be summarized in three steps: 1- binding of the amyloid protein to the plasma membrane of a brain cell through a primary interaction with a ganglioside (GM1 or GM3) followed by 2- cholesterol-assisted insertion and 3- oligomerization into a Ca2+ permeable pore (Di Scala et al., Sci Rep. 2016, 6: 28781).
Elucidating this universal mechanism allowed us to create the first inhibitor of amyloid pore formation, now called AmyP53, that is active against wild-type and mutant forms of the proteins involved in Alzheimer's and Parkinson's diseases. A full description of the anti-pore properties of this inhibitor has been published here.
Disruptive technology
AmyP53 is not only one of the first molecules that target the oligomer cascade, it is the only one that can block the formation of the neurotoxic oligomers in the plasma membrane of brain cells.
Therapeutic implications:
Reversibility of Aβ oligomer neurotoxicity: Insights into the treatment of Alzheimer’s disease

43000 visitors milestone reached!
Publications
What Is life? Rethinking Biology in Light of Fundamental Parameters.
Emergence of a second SARS-CoV-2 variant with a tremendous genetic leap from its ancestors.Colson P, La Scola B, Beye M, Delerce J, Raoult D, Fantini J.J Med Virol. 2023 Oct;95(10):e29124.
Host Membranes as Drivers of Virus Evolution.Matveeva M, Lefebvre M, Chahinian H, Yahi N, Fantini J.Viruses. 2023 Aug 31;15(9):1854. doi: 10.3390/v15091854.
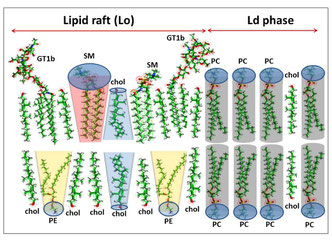
Lipid rafts and human diseases: why we need to target gangliosides.
Fantini J. FEBS Open Bio. 2023 Apr 13. doi: 10.1002/2211-5463.13612. Online ahead of print.PMID: 37052878
Fantini J, Azzaz F, Chahinian H, Yahi N.Viruses. 2023 Jan 19;15(2):284. doi: 10.3390/v15020284.
Fantini J, Chahinian H, Yahi N.Int J Mol Sci. 2023 Jan 18;24(3):1923. doi: 10.3390/ijms24031
Structural basis of botulinum neurotoxin serotype A1 binding to human SV2A or SV2C receptors.
Azzaz F, Hilaire D, Fantini J.Chem Biol Interact. 2023 Mar 1;373:110384. doi: 10.1016/j.cbi.2023.110384.
Azzaz F, Chahinian H, Yahi N, Fantini J, Di Scala C.Int J Mol Sci. 2023 Jan 16;24(2):1760. doi: 10.3390/ijms24021760.